Australian Genomics has updated the previously released clinical consent package (2019) to provide practice-informed clinical consent forms for genomic and genetic testing. These materials, released in 2024, meet current national standards and clinical practices. This revised package includes a health professional guide and patient fact sheet.
Over the next year, Australian Genomics will progress efforts to co-develop resources to support Aboriginal and Torres Strait Islander people through the consent process, along with customised consent forms for somatic testing for non-inherited genetic conditions and prenatal genomic testing.
Download our latest clinical consent form and supplementary information below.
Please ensure you have appropriate approval(s) if you decide to use these forms in your clinical practice.
Tissue-targeted (somatic) testing consultation
Australian Genomics has developed a clinical tissue-targeted (somatic) genomic consent package in collaboration with an expert working group. This comprehensive package reflects current regulatory requirements and best practices, aiming to improve the clarity, consistency, and documentation of patient consent in tissue-targeted genomic testing.
Resources
Tools for patients
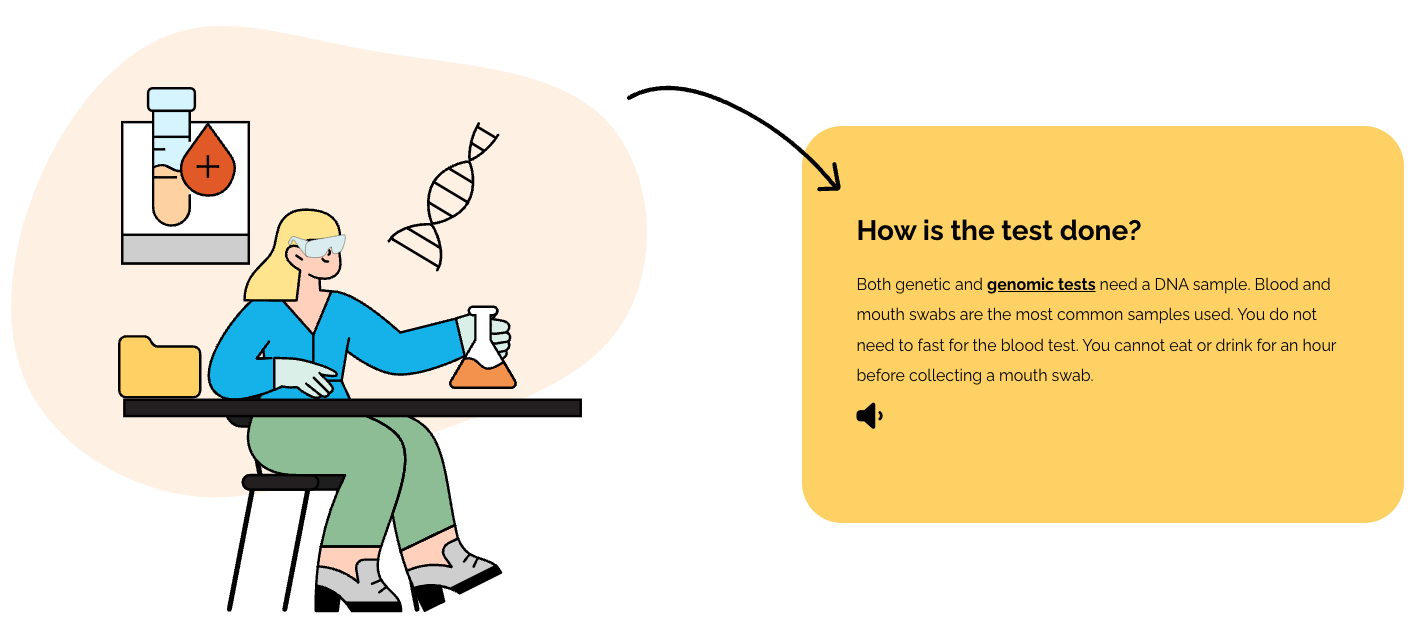
Genomic testing can be complicated. This interactive online tool provides easy and accessible information in bite-sized chunks to guide patients through the key concepts of genomic testing. This tool is designed to complement the updated clinical consent package above.
Contact
Keri Finlay

