In June 2018, Australian Genomics ran a public consultation to gather feedback on a proposed national clinical consent package for genomic testing.
The ‘package’ is made up of a national clinical consent form for genomic testing and a supporting information hand-out.
Why a national clinical consent form for genomic testing?
Genomic testing raises complex issues, with a number of possible testing outcomes and potential implications for the individual and their family.
Providing patients with clear information during the consent process for genomic testing is essential to support well-informed decisions about testing.
Genetic testing services across Australia currently take independent approaches to clinical genomics consent. This can be a barrier to the flow of health information across services and can confuse the interpretation of laws and policies about sharing genomic data for clinical or research purposes.
Australian Genomics formed a National Clinical Consent Working Group to identify opportunities to standardise consent processes for genomic testing across the country. Using a consultative approach with state and territory-based genetics services, the Group arrived at a single clinical genomic consent form and accompanying information (the consent package) that can be used nationally for genetic conditions.
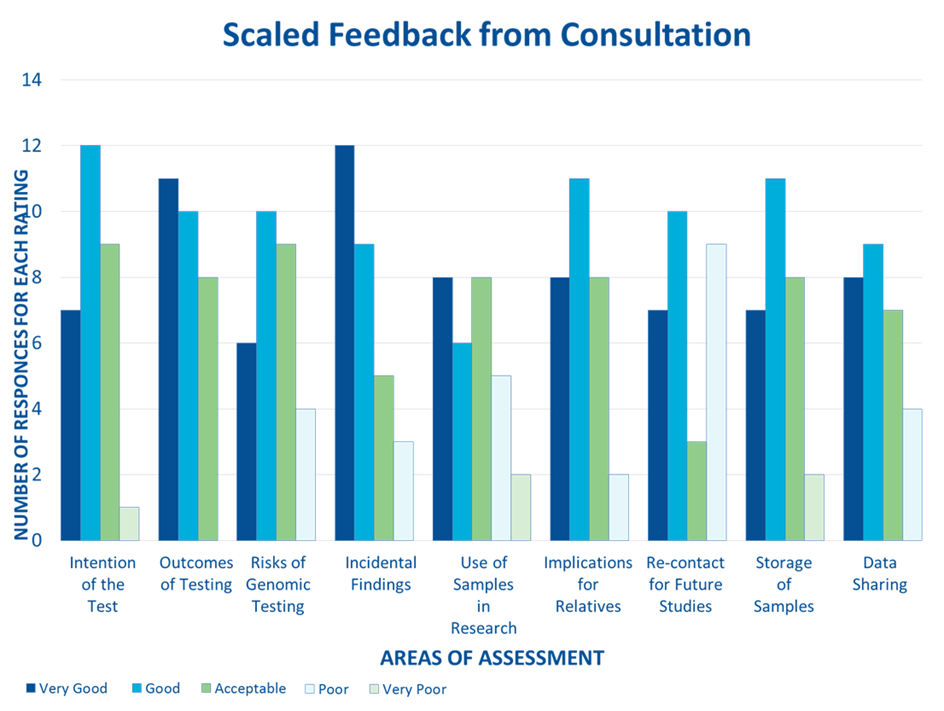
What feedback did we get during the consultation?
Over 80% of respondents found all elements in the consent form and supporting documentation – with the exception of ‘re-contact for future studies and ‘use of sample for research’ – either very good, good or acceptable.
See details below.
This feedback was reviewed by the National Consent Working group and the consent form and supporting documentation modified to incorporate much of the feedback received.
A final targeted review of the consent package is underway with a small group of organisations & experts, as recommended by respondents to the consultation.
More to come soon!
For more information please contact Working Group coordinator, Keri Pereira at keri.pereira@vcgs.org.au