Digital technologies are increasingly being sought to manage the research consent process. CTRL is a secure web-based application that gives research participants the opportunity to engage more with a research program, update their personal details and choices about being involved in research, and take the lead in decision-making about secondary use of their genomic and health information.
CTRL also benefits research organisations. Electronic consent records replace paper and are more easily retrieved, it is interoperable with study databases like REDCap and manages permissions for secondary use of data using internationally endorsed standards.
CTRL has undergone recent upgrades and is now freely available to be adopted by the research community.
What's coming next for CTRL?
Australian Genomics’ online dynamic consent platform CTRL has undergone a major upgrade in collaboration with the Garvan Institute of Medical Research. It includes a complete code re-write, a modern interface and a scalable backend. In coming months CTRL will introduce a number of other features to simplify workflows, enhance participant engagement, and provide research teams with powerful insights.
In the upcoming months, CTRL will introduce advanced consent transition management, automated participant communication, real-time dashboards, and advanced integrations with REDCap and Elsa.
Current capabilities
CTRL provides a comprehensive dynamic consent solution for both participants and research teams. With the Participant Portal, individuals can easily provide, review, and update their consent preferences at any time, ensuring they remain actively engaged throughout a study. Automated consent capture reduces the need for manual documentation by digitally collecting and storing consent, streamlining the administrative process for research teams. Every consent decision is securely logged through audit logging ensuring transparency and traceability. Additionally, CTRL is designed to be flexible, functioning as a standalone solution with its built-in database or integrating with platforms like REDCap for seamless data syncing.
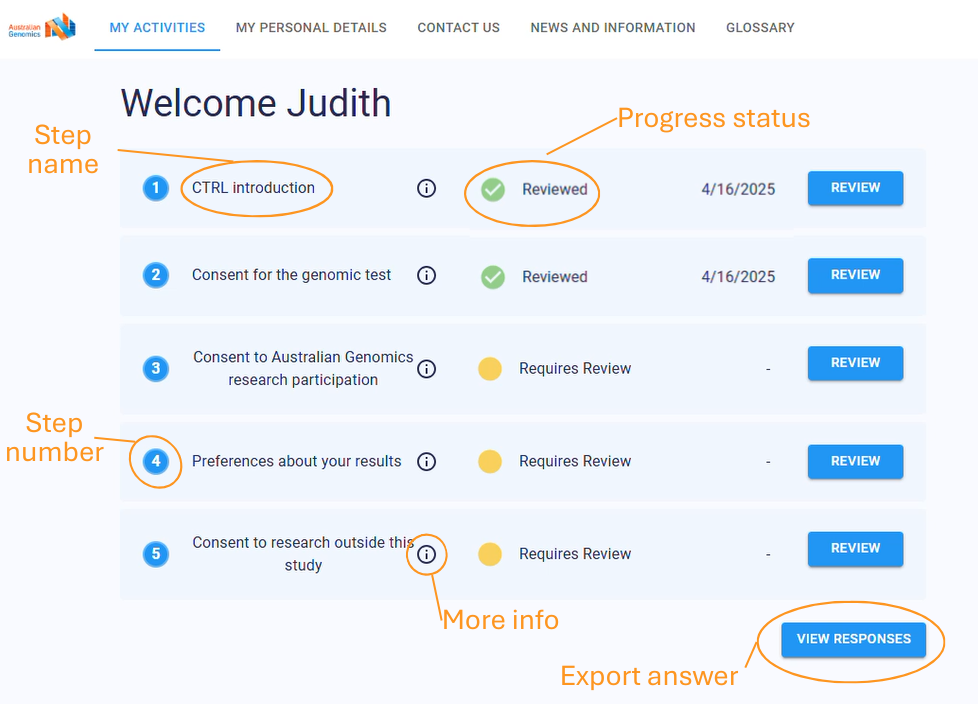
Upcoming features
Several new features are being introduced:
- Enhanced Consent Transition Management: Improved tools for tracking changes in participant consent over time, ensuring researchers always have the most up-to-date permissions. (e.g. child participant transition to adulthood).
- Automated Participant Communication: Scheduled updates and notifications will keep participants informed and engaged throughout the study.
- Real-Time Dashboards & Data Insights: Research teams will have instant access to consent metrics, participant engagement data, and workflow analytics.
- Expanded Integrations: Deeper connectivity with REDCap and the planned integration of Elsa will allow for seamless data management across multiple research platforms.
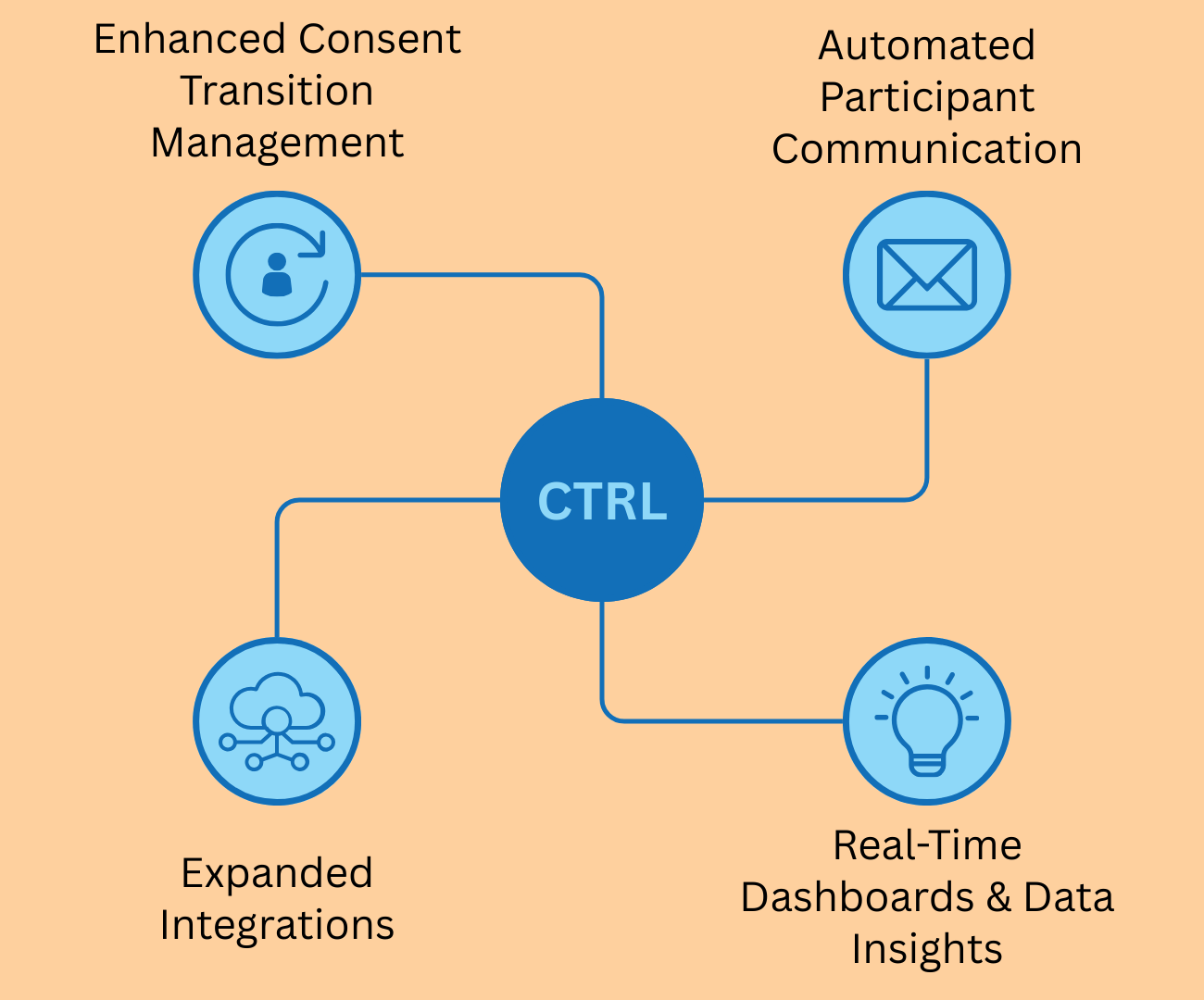
A more efficient admin portal
A major focus of this update is the revamped Admin Portal, designed to simplify consent management for research teams. The improved portal will feature real-time monitoring tools that allow research teams to track participant registrations, consent completions, and changes at a glance. Automated alerts and summary reports will provide configurable notifications to keep teams updated on critical consent modifications. Enhanced security features, including two-factor authentication and granular permission controls, will further strengthen data protection.
These updates will make consent management more efficient, reduce administrative workload, and secured.
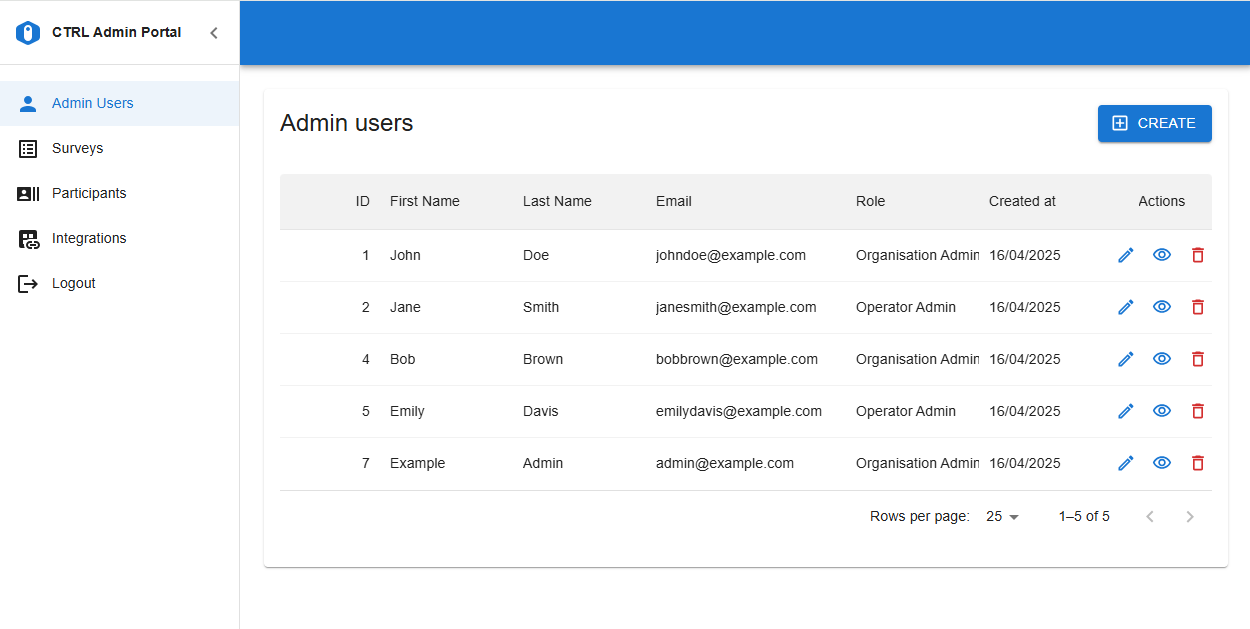
New modern user interface to manage Admin Users, Participants and Surveys
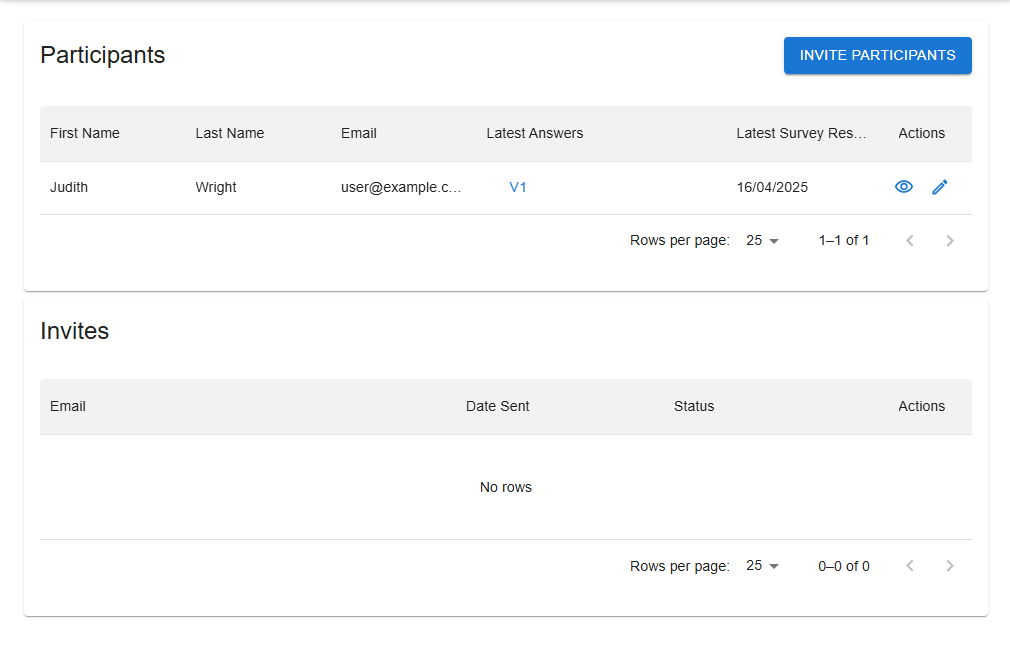
Invite, view and manage participants and keep track of invitations
CTRL demonstration
Access a demonstration version of CTRL using the generic Study ID on the registration page: A1234123
See full instructions for access.
Watch a video walkthrough below.
Resources
Read the dynamic consent evidence summary.
Read the European Journal of Human Genetics paper on how we designed and developed CTRL.
Contact
Matilda Haas

